Ginkgo Bioworks (NYSE:DNA) is a leading synthetic biology company that went public through a SPAC in 2021. A large amount of hype has surrounded Ginkgo and they managed to catch the tail end of a red-hot IPO market, providing them with a relatively large market capitalization despite their modest achievements. Even after an 80%+ decline in share price, the stock remains highly speculative and could decline further on market weakness or if the company stumbles.
Ginkgo Bioworks
Ginkgo is aiming to make biology easier to engineer with a horizontal platform that they believe will eventually make biology programmable in a manner analogous to computers. The belief that biology is code lies at the heart of Ginkgo and their business model, which aims to make Ginkgo the AWS or app store of biology.
From the outset it should be stated that the DNA is code analogy is not necessarily accurate or even that useful. Even if it were an accurate analogy, it is not clear that DNA is close to being well enough understood to be treated like deterministic code. Xenobots are just one example of how complex biology is and how DNA is only one determinant of function. The genome determines the hardware that cells have (proteins, signaling components, computational components) but stimuli can change how cells behave. Structure and function are driven by a kind of software that is based largely on developmental bio-electricity. For example, by changing electrical patterns in the tissue of flatworms, the flatworm can be cut in half and will regrow a second head rather than a tail, all without changing the DNA. Similarly, after a salamander loses a limb, the cells work together to regrow the limb. This type of complex system level behavior is still poorly understood.
Synthetic biology using yeast and other standard hosts involves two phases:
Ginkgo has created a horizontal platform for programming cells across organisms and makes this platform available to customers who want to program cells for applications in food, medicine, cosmetics, agriculture, etc. Ginkgo has chosen to focus solely on its core competencies (discovery and engineering), driven by a belief that this is where synthetic biology creates value, rather than further downstream. They have spent almost half a billion dollars building highly automated labs with advanced diagnostic tools that can be used to design, build and test genes that are added to microorganisms or other cells. Ginkgo claims to be able to create 50,000 different genetically modified cells a day, with the Foundry's primary output being organisms that efficiently convert feedstock into a desired molecule.
At the end of a program, customers receive the engineered organism along with a plan for manufacturing and downstream processing. Although this raises questions about how well strains initially perform at scale, how much ongoing assistance Ginkgo provides to customers to help them continue lowering production costs and what happens in the event that there are manufacturing issues.
Ginkgo's Foundry provides significant economies of scale, with output being scaled 3x annually since 2015 and the average cost per unit of operation falling approximately 50% annually. These cost savings are passed on to customers with the aim of stimulating demand.
Economies of scale are essential to Ginkgo's ambitions to develop the dominant horizontal platform in the industry. Economies of scale refer not just to cost, but also the capabilities of the platform. If Ginkgo's value proposition simply ends up being that they can engineer organisms at a lower cost than competitors their platform will capture little of the value it creates. Customers will not give away large shares of downstream value just to lower R&D costs slightly, particularly if they expect a large amount of product revenue. To realize their vision, Ginkgo must be able to perform R&D services that are beyond the capabilities of competitors.
Foundry
Ginkgo's Foundry is a large and efficient biology lab which makes extensive use of automation and proprietary workflows. It is paired with Ginkgo's Codebase to improve the productivity of scientists programming cells. Functions performed in the Foundry include organism engineering, design, DNA synthesis and assembly, genome engineering, protein engineering and characterization, transformation and transfection, next generation sequencing, assay development, ultra-high throughput screening, analytical chemistry, synthetic chemistry, directed evolution and fermentation.
Creating a molecule is a multi-step process, with each step requiring an enzyme. Synthetic biology companies must optimize the entire pathway and if there is an issue with one of the steps, correct it. This means that synthetic biology companies are trying to optimize across a massive search space, making machine learning and high-throughput / low cost lab operations a necessity. Molecules are not always the objective of a program though with Ginkgo also targeting the production of organisms, like in their nitrogen fixation program.
Reducing lab expenses is at the heart of Ginkgo's business and is achieved through automation, parallel processing and miniaturization. Through these types of measures, as well as an increased footprint, Ginkgo has been able to scale its lab operations by orders of magnitude over the past decade.
Ginkgo has observed that Foundry capacity, as determined by the number of strain tests, has historically increased 3-4x annually through a combination of increased footprint and productivity. Going forward Ginkgo expects to increase their physical footprint 25-30% annually between 2021 and 2025 and improve productivity through:
Due to improvements in technology and processes, along with increasing scale, Ginkgo believes their Foundry costs are now several times lower than the status quo (manual lab work), an advantage that will only grow over time.
Parallelization - Many biotechnology tools place operations in parallel to increase throughput. This is the principal by which the cost of DNA synthesis and sequencing has been reduced so dramatically. Parallelization of operations is also utilized in gene editing and screening.
Automation - Automation is used to minimize labor in tasks like liquid handling. Ginkgo employs both off-the-shelf and proprietary liquid handling techniques. For example, the Foundries transfer minute amounts of liquid reagents under positive pressure using rotary valves. Ginkgo also employs automation technology that it co-developed with Transcriptic (now Strateos). The importance of robotics is probably overestimated though. Similar setups can be found in many manufacturing plants globally and the use of robotics will not necessarily lead to a competitive advantage, particularly if competitors have access to the same capabilities.
Miniaturization - Costs are also reduced through miniaturization, such as conducting fermentation in wells on a plate and then scaling up production of more promising cells. Increasing the number of wells on a plate is then a simple way to increase throughput. The industry standard was 96 samples, but Ginkgo moved to 384 and then 1,536 wells plates. There are still potentially large gains to be made through miniaturization, which taken to the extreme could involve the manipulation and monitoring of single cells.
Ginkgo is not alone in pursuing these types of initiative though, companies like Amyris (AMRS) and Intrexon have been working on high throughput synthetic biology for many years. What differentiates Gingko is the extent to which they are pursuing scale, parallelization, miniaturization and automation. The degree to which they are differentiated is not clear though, given that many of their capabilities come from third-party vendors.
Third-Party Suppliers
DNA synthesis is an integral part of synthetic biology and while Ginkgo largely relies on Twist Bioscience (TWST), they also acquired synthesis capabilities through Gen9 in 2017. Gen9's IP enables Ginkgo to synthesize large (>10 kb) genetic constructs that contain many genes, up to entire metabolic pathways. In comparison, companies like Twist synthesize DNA up to approximately 300 b in length. If longer pieces of DNA are desired, they must be stitched together, a time-consuming process that can introduce errors. Ginkgo can directly synthesize large constructs rapidly and to their exact specifications, but it is unclear how cost competitive this. Given how much Ginkgo spends with Twist, it is likely that the vast majority of their DNA requirements are met externally. Twist is tightly integrated with Ginkgo to meet their volume and turnaround requirements and is a leader on cost.
Fermenters and automated liquid handlers are typically supplied by Sartorius and Hamilton, respectively.
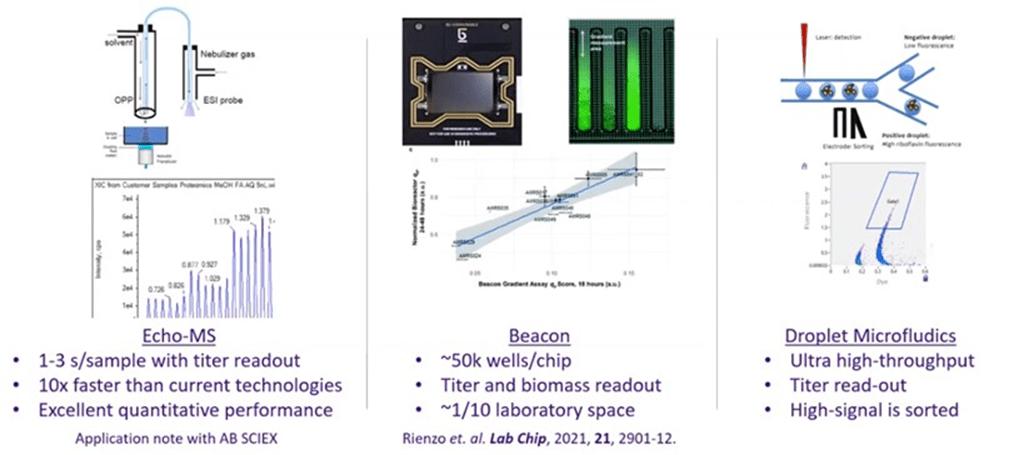
Ginkgo has developed custom functional assays for cell characterization in collaboration with Berkeley Lights (BLI). Berkeley Lights' platform utilizes fluctuations in an electric field to move single cells. Berkeley Lights' stock has been under pressure over the past 12 months due to a combination of valuation concerns, relatively poor financial performance and questions regarding product-market fit. Technology in the high-throughput screening space is still evolving rapidly and it is not clear which technology will ultimately become dominant.
Screening technologies can be classified by throughput and measurement quality / information content. Increased throughput often comes at the expense of measurement quality / information content. Amyris is currently using the technologies in brown in Figure 6 and is exploring the technologies in blue. Compared to Berkeley Lights' technology, droplet-based microfluidics could potentially offer significantly greater throughput and Echo-MS and Rapid-Fire MS could offer similar throughput with higher measurement quality.
As the capabilities of these types of technologies improve, so will the ability of synthetic biology companies to rapidly produce organisms that can cost-effectively manufacture target molecules at scale.
Another differentiator for Ginkgo is their extensive use of long-read sequencing technologies from Pacific Biosciences (PACB) and Oxford Nanopore (OTC:ONTTF). Open-source databases primarily consist of short-read sequencing data but many microbial genomes (e.g., bacteria) are highly repetitive, making them difficult to analyze with short-read sequencing. Utilizing long-read sequencing allows Ginkgo to generate higher quality genome assemblies. PacBio's accuracy can be leveraged to faithfully reconstruct diploid (e.g., mammalian) genomes, capture ultra-low-frequency mutations, and sequence through complex alleles. Long-read sequencing platforms are also adept at analyzing gene isoforms. In addition, Oxford Nanopore's technology is good at capturing epigenetic information and linking the behavior of regulatory elements such as distal enhancers and promoters.
Codebase
Ginkgo's Codebase consists of reusable pieces of genetic code and engineered cell lines, which Ginkgo expects to drive performance improvements over time. Biology provides a massive search space that is still largely unexplored, particularly at genome-scale, making incremental data valuable. Machine learning and past experience can be leveraged to more efficiently search this massive design space.
Ginkgo's Codebase contains 3.4 billion unique gene sequences pulled from all public databases and 440 million proprietary sequences that they have acquired. Zymergen (ZY) is believed to have a larger and more diverse codebase than Ginkgo, although comparable data on this is scarce. The Codebase is also believed to be largely sequences without functional data, meaning that for most sequences it is not clear what, if any, protein it encodes for. A DNA sequence alone is generally insufficient to make definitive predictions of an organism's traits though, as DNA does not deterministically dictate function. At each stage of biology's central dogma there are complex processes which are not fully understood, like alternative splicing, post-translational modifications and subcellular localization.
Ideally synthetic biology companies would be strain agnostic, but this is difficult in practice as transferring tools between strains is not straightforward. Ginkgo's familiarity with a range of hosts is often touted as one of their strengths. Yeast is probably the most commonly used organism for Ginkgo's strain engineering projects, but bacteria, fungi and mammal cells have also been used. Yeast is well-understood, relatively easy to manipulate and post-translational modifications are possible. Ginkgo has developed novel synthetic promoters (sequences that turn on the expression of a gene of interest) that allow them to increase production of proteins in yeast. They tested thousands of designs to select a small number of promoters with high performance which can be used in any program producing a protein in yeast.
In the long-run an improved understanding of cells could lead to rational design. Physics engines for cells are improving, which is helping to improve the understanding of cells. Continued advances in machine learning, molecular simulation and other computational techniques could also improve the ability to program cells and Ginkgo's Foundry is positioned to build the datasets required for such a computational approach. Computational approaches would reduce experimental burden in the future, lowering costs further and freeing Foundry capacity to work on increasingly complex cell engineering challenges.
Ginkgo's business model is based around the belief that data collected from past programs will provide a competitive advantage, but the competitive advantage provided by data is often poorly understood. Depending on the application, new data can continue to improve performance even at a massive scale. In other applications performance may begin to peak after a relatively small volume of data has been collected. For synthetic biology these dynamics aren't clear, but given the size of the search space it is likely data will continue to drive performance improvements, even at a massive scale. This dynamic will also be heavily dependent on the tools available to collect and analyze data. Exponential improvements in tools and a corresponding decline in costs make it easier for new entrants to scale-up to Ginkgo's size.
Ginkgo's key differentiator should be its ability to engineer new metabolic pathways, which open up design options. Their ability to do this effectively remains unclear at this stage though. For example, they have shown they can engineer a new pathway in cannabinoids which avoids existing IP, but this does not appear to have been commercially viable as Cronos (CRON) recently licensed IP from Aurora Cannabis (ACB). In comparison, Amyris utilized the same pathway protected by Aurora's IP but claim to have achieved a workaround based on a technicality that provides freedom to operate.
Scale-Up
Scale-up remains the most difficult aspect of synthetic biology for a number of reasons, including a looming capacity bottleneck. Gingko's decision to avoid commercial production could be problematic as it provides competitors with a potential source of advantage. It may also mean that Ginkgo chooses to primarily target higher value add products where scale-up is less of a concern (e.g. pharma).
While Ginkgo has chosen not to become involved in scale-up directly, they have introduced measures to ensure that their programs can be executed at scale. This is one area where the use of royalties or equity are important to ensuring that the incentives of Ginkgo and their customers are aligned. Gingko has built relationships with a number of leading contract manufacturing organizations and demonstrated that they can transfer lab-developed protocols to commercial scale (50,000+ L fermentation tanks) with predictable performance. Ginkgo also has an in-house deployment team dedicated to supporting the scale-up of customer operations. Ginkgo has shared one example in which fermentation volume of 250 mL was effectively translated into pilot and commercial scale.
Simply achieving commercial scale is insufficient though. If multiple companies are producing the same molecule, the lowest cost producer will reap most of the benefits. Vertically integrated strain engineering and commercial fermentation provides the opportunity to lower costs through the optimization of variables like fermentation conditions, strain performance at scale, fermentation facility design, downstream processing and waste valorization.
Conclusion
Ginkgo currently has the leading synthetic biology platform, but its advantage over competitors is unclear. An ex-Ginkgo executive reportedly stated that half of their projects fail at the front end and the other half at fermentation/scale-up (past programs have reached commercial production, meaning this is hyperbole). Many competitors with far less resources have shown the ability to successfully commercialize products and Ginkgo's low past success rate probably speaks more to the type of projects they have taken on than the capabilities of their platform. Failure during strain engineering is more likely if the target molecule or metabolic pathway is complex. In these types of cases, even if strain engineering succeeds it may only be possible to manufacture the target molecule in trace amounts.
While Gingko has tremendous potential, it still faces a number of obstacles, like manufacturing handoff, production scale-up, related-party revenue concentration, business model uncertainty and potential manufacturing bottlenecks. Even if Ginkgo becomes the dominant platform in the industry, they may not be able to translate this into significant shareholder value. Ginkgo's business model is often held up as the company's most attractive attribute, but pursuing a horizontal strategy in a nascent industry probably doesn't make sense. This is an issue that is not well understood and one I will cover in a separate article.